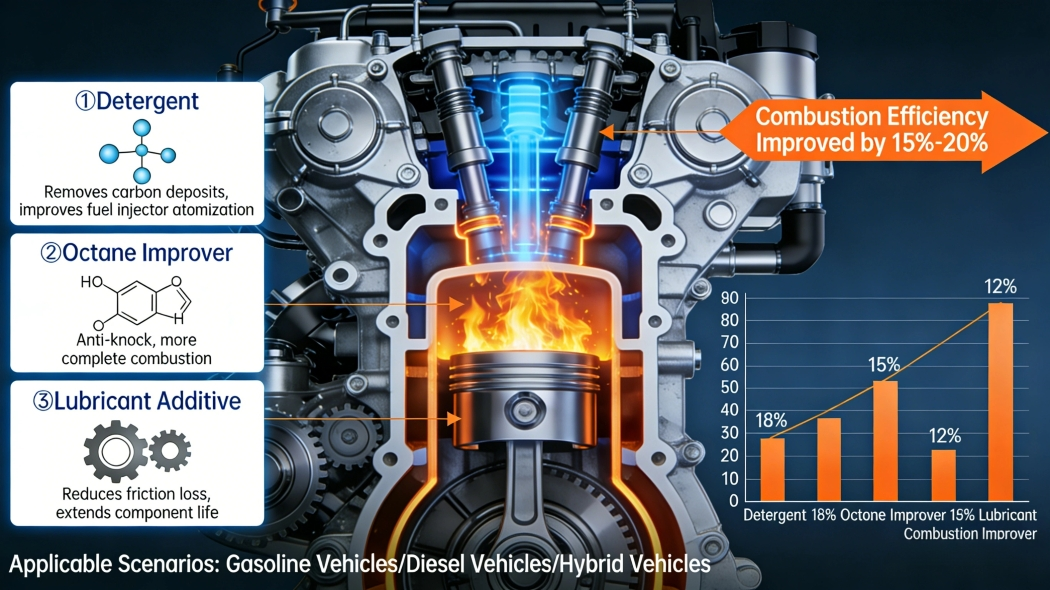
Oxygenated Fuel Additives: Enhancing Complete Combustion
Mechanism: How Ethanol and 1-Butanol Increase Oxygen Availability and Reduce CO/HC Emissions
Both ethanol (C2H5OH) and 1-butanol (C4H9OH) have oxygen in their molecules, which means they bring extra oxygen right into the engine when mixed with regular fuel. The added oxygen helps burn the fuel more completely, cutting down on those nasty leftover combustion products we all hate. When compared straight up against plain old gasoline, mixtures containing these alcohols cut carbon monoxide emissions anywhere from 20 to 30 percent, and reduce unburned hydrocarbons by around 15 to 25 percent. This happens because the fuel burns cleaner and more thoroughly in most engines during normal operation conditions. Pretty impressive for something that sounds so technical!
Efficiency Trade-offs: Balancing Brake Thermal Efficiency Gains Against NOx Formation
Adding oxygenated compounds to fuels typically boosts brake thermal efficiency somewhere between 3 and 8 percent because they help burn fuel more completely. But there's another side to this coin that engineers need to watch out for. When combustion temperatures spike up, it actually speeds up the production of thermal NOx through what's known as the Zeldovich mechanism. Research shows something interesting happening here too: whenever thermal efficiency goes up about 10 percent thanks to ethanol based oxygenates, NOx emissions tend to jump anywhere from 12 to even 18 percent higher. So meeting emission standards isn't simply about throwing in some additives. Mechanics really have to fine tune things carefully looking at how much additive gets used, when exactly it's injected into the system, and making sure engines are properly calibrated overall. Just adding stuff randomly won't cut it these days.
Nanoparticle Catalysts: Boosting In-Cylinder Reaction Kinetics
Nanoparticle catalysts represent a frontier in combustion optimization, where materials like aluminum oxide (Al₂O₃) and cerium dioxide (CeO₂) act as molecular-level combustion promoters. Their ultra-high surface area-to-volume ratio creates abundant active sites that accelerate key oxidation and soot-removal reactions through surface catalysis pathways.
Al₂O₃ and CeO₂ Nanoparticles as Combustion Promoters: Surface Catalysis and Soot Oxidation Pathways
Aluminum oxide nanoparticles boost how flames spread because they grab onto those pesky hydrocarbon radicals, which basically cuts down the energy needed to get oxidation going. On the other side of things, cerium dioxide has this neat trick where it stores oxygen and then releases it when there's plenty of fuel around, only to suck it back in again when conditions are leaner. These two effects working together cut particulate matter emissions somewhere between 15 and 30 percent in diesel engines. Plus, the combustion process becomes just a little bit more efficient since everything burns more completely. For manufacturers dealing with emission regulations, these improvements represent real value despite being relatively small efficiency boosts.
Practical Challenges: Dispersion Stability, Agglomeration, and Real-World Fuel Economy Validation
Getting those impressive lab results with nanoparticles translated into actual fuel additives for everyday use is still pretty tough. When these particles clump together during storage or when things get hot, they lose their effectiveness because there's just less surface area available for reactions. And if they don't spread out properly in the fuel system, it can lead to problems like clogged injectors down the road. Most engineers working on this are trying different approaches right now, mainly looking at ways to keep the particles stable using special chemicals and techniques like sound waves to mix them better. What we've seen from tests with real vehicle fleets tells another story though. Even though nanoparticles work well in controlled environments, their performance drops somewhere between 8% to 12% when put through the wringer in older engines that deal with all sorts of fuel qualities and driving conditions. This gap highlights why proper field testing needs to happen long before anyone starts selling these products commercially.
Ignition Modifiers: Optimizing Combustion Timing for Maximum Efficiency
Fuel additives that modify ignition timing are engineered to enhance combustion efficiency by precisely controlling when fuel ignites relative to piston position. By advancing or delaying ignition onset, these compounds help engines operate closer to thermodynamic limits—maximizing energy extraction while minimizing waste heat and emissions.
Cetane Improvers (e.g., 2-Ethylhexyl Nitrate) and Diesel Ignition Delay Reduction
Cetane boosters like 2 ethylhexyl nitrate (2 EHN) work by breaking down into free radicals when subjected to intense heat and pressure inside diesel engines. What happens next is pretty interesting actually. The breakdown process speeds up autoignition which makes starting those engines much easier when it's cold outside. Tests show this can cut carbon monoxide and hydrocarbon emissions by around 15 percent during colder operations. But there's a catch though. When the ignition delay gets shortened so much, the pressure inside the cylinders spikes dramatically. And if the injection system isn't properly adjusted for this change, nitrogen oxide emissions tend to go up between 8 and 12 percent instead. That's why proper tuning remains absolutely critical for maintaining emission control benefits while using these additives.
Octane Enhancers (e.g., MMT) Enabling Higher Compression Ratios in SI Engines
Spark ignition engines benefit from something called methylcyclopentadienyl manganese tricarbonyl, commonly known as MMT. What this substance does is stop engine knocking by keeping fuel oxidation stable during those initial combustion stages. As a result, manufacturers can safely bump up compression ratios by about 1.5 to 2 points, which leads to better brake thermal efficiency gains ranging between 4% and 7%. Real world testing shows cars using these higher octane fuels actually produce around 5% fewer carbon dioxide emissions for every kilometer they travel. However, there are limits to how much manganese can be used because too much builds up over time on important engine components like oxygen sensors and catalytic converters, which is why most regulations cap the allowable dosage levels.
FAQ Section
What are oxygenated fuel additives?
Oxygenated fuel additives are compounds like ethanol and 1-butanol that contain oxygen within their molecular structure. They are mixed with regular fuel to enhance combustion efficiency and reduce emissions.
How do nanoparticle catalysts work in combustion engines?
Nanoparticle catalysts, such as aluminum oxide and cerium dioxide, improve combustion by providing abundant active sites that accelerate oxidation and soot-removal reactions, resulting in cleaner emissions.
What are the challenges of using nanoparticle catalysts?
The main challenges include ensuring the stable dispersion of nanoparticles to prevent clumping, and validating their performance in real-world fuel systems to maintain effectiveness.
How do ignition modifiers optimize combustion?
Ignition modifiers control the timing of fuel ignition relative to piston position, allowing for more efficient combustion and minimizing waste and emissions.